R&D
Nonghyup Chemical Research Center

R&D Goals

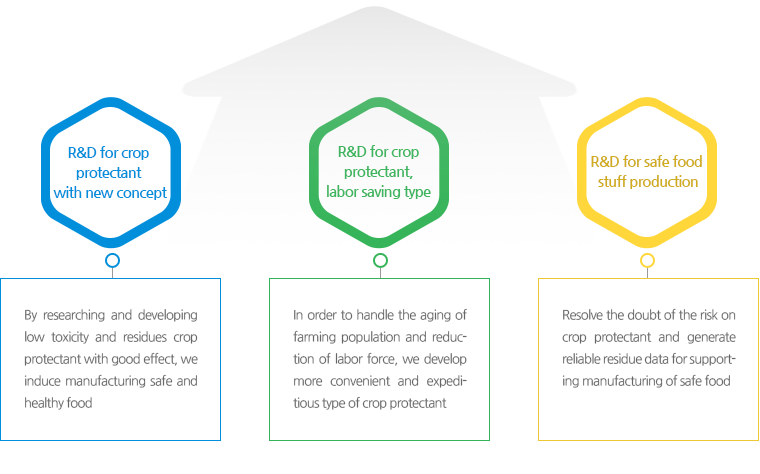
-
GLP(Good Laboratory Practice)
- - An international standard to secure the reliability of non-clinical tests conducted to evaluate the safety of crop protectant, medical products and chemical substances, etc.
- - Systematic management of all issues related to the testing procedures including organization, operation, facilities, and execution and inspection of the tests performed by the testing institution
- • Establishment of an operation system that complies with the regulations of the GLP management of OECD (Standard work guideline)
- • Facilities: ‘GLP test and research building’ to perform GLP testing (total floor area of 700 pyeong (approximately 2,300m2)
- • Organization: Secure outstanding personnel with many years of accumulated experiences in each testing field
